Learn more about the Irbesartan-Authorized Generics
General Considerations
AVAPRO may be administered with other antihypertensive agents and with or without food.1
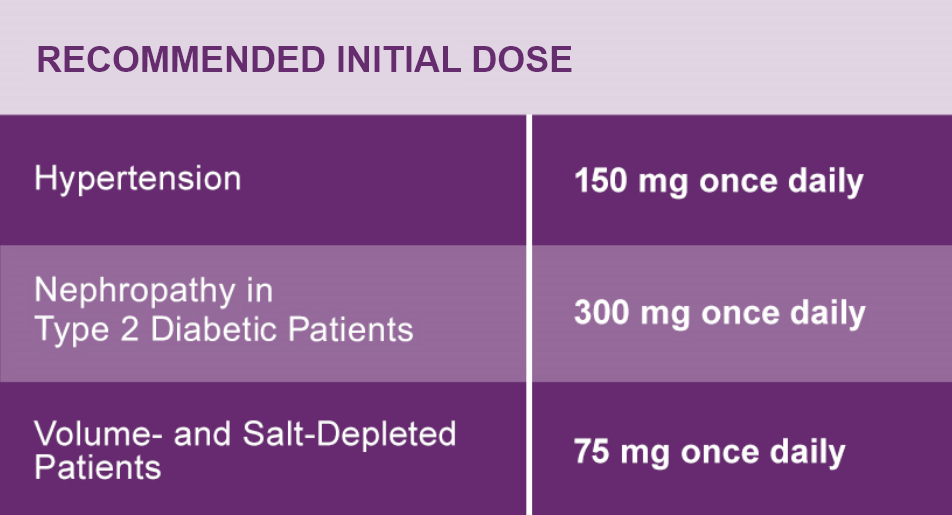
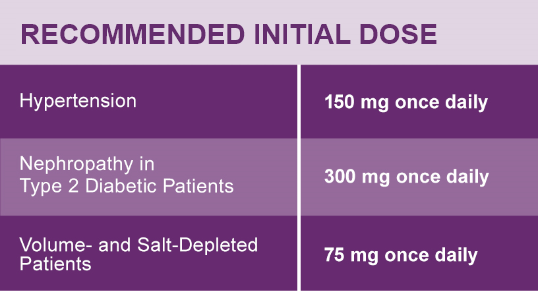
Hypertension
The recommended initial dose of AVAPRO is 150 mg once daily. The dosage can be increased to a maximum dose of 300 mg once daily as needed to control blood pressure.1
Nephropathy in Type 2 Diabetic Patients
The recommended dose is 300 mg once daily.1
Dose Adjustment in Volume- and Salt-Depleted Patients
The recommended initial dose is 75 mg once daily in patients with depletion of intravascular volume or salt (e.g., patients treated vigorously with diuretics or on hemodialysis). Symptomatic hypotension may occur after initialization of treatment with AVAPRO in volume or salt-depleted patients. Correct volume or salt depletion prior to administration of AVAPRO.1
Dosage Forms and Strengths
AVAPRO 75 mg is a white to off-white biconvex oval tablet debossed with a heart on one side and ″2871″ on the other.1
AVAPRO 150 mg is a white to off-white biconvex oval tablet debossed with a heart on one side and ″2872″ on the other.1
AVAPRO 300 mg is a white to off-white biconvex oval tablet debossed with a heart on one side and ″2873″ on the other.1
General Considerations
The side effects of irbesartan are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter.2
Maximum antihypertensive effects are attained within 2 to 4 weeks after a change in dose.2
AVALIDE may be administered with or without food.2
AVALIDE may be administered with other antihypertensive agents.2
Renal Impairment
The usual regimens of therapy with AVALIDE may be followed as long as the patient’s creatinine clearance is >30 mL/min. In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so AVALIDE is not recommended.2
Hepatic Impairment
No dosage adjustment is necessary in patients with hepatic impairment.2
Add-On Therapy
In patients not controlled on monotherapy with irbesartan or hydrochlorothiazide, the recommended doses of AVALIDE, in order of increasing mean effect, are (irbesartan-hydrochlorothiazide) 150/12.5 mg, 300/12.5 mg, and 300/25 mg. The largest incremental effect will likely be in the transition from monotherapy to 150/12.5 mg.2
Replacement Therapy
AVALIDE may be substituted for the titrated components.2
Initial Therapy
The usual starting dose is AVALIDE 150/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum of 300/25 mg once daily as needed to control blood pressure. AVALIDE is not recommended as initial therapy in patients with intravascular volume depletion. Volume- or sodium-depletion, should be corrected prior to administration of antihypertensive therapy.
Dosage Forms and Strengths
AVALIDE® (irbesartan-hydrochlorothiazide) 150/12.5 mg and 300/12.5 mg film-coated is peach, biconvex, and oval with a heart debossed on one side and ″2875″ or ″2876″ on the reverse side, respectively.2

Learn more about the Irbesartan-Authorized Generics
References
1. AVAPRO [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2018.
2. AVALIDE [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2018.